| BeschreibungDNA damaged by carcinogenic 2-aminofluorene AF.jpg |
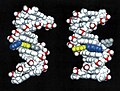
Structures of DNA damaged by the carcinogenic aromatic amine 2-aminofluorene (AF). Left: AF in the B-DNA major groove, the predominant structure at a mutational coldspot. Right: AF inserted into the helix with displacement of the damaged guanine, the predominant structure at a mutational hotspot. Color code: AF: blue; AF-damaged guanine: yellow; cytosine partner to damaged guanine: gray.
Molecular Understanding of Mutagenicity
Brian E. Hingerty, Oak Ridge National Laboratory
Suse Broyde, New York University
Dinshaw J. Patel, Memorial Sloan Kettering Cancer Center
Research Objectives
To elucidate why certain DNA base sequences are mutational hotspots when damaged by carcinogenic environmental chemicals.
Computational Approach
Molecular mechanics calculations in combination with data from NMR experiments in the form of distances between hydrogens on the carcinogen-damaged DNA molecule are employed to produce molecular views of the damaged DNA that are in agreement with the data. The computations are carried out with the molecular mechanics program DUPLEX on the Cray C90.
Accomplishments
The aromatic amines are a category of environmental carcinogens present in tobacco smoke, automobile exhaust, dyes and other industrial products, and broiled meats and fish. These substances, when activated biochemically, can bind to DNA and subsequently cause a mutation when the DNA replicates. Such mutations are widely believed to be the initiating event in carcinogenesis by these substances. Often, the target base in the DNA to which the carcinogen binds is guanine (G). Interestingly, it has been found that a carcinogen-bound guanine may be highly mutagenic (a hotspot) or weakly or non-mutagenic, depending on what the neighbor bases are. One example of such a sequence that has been of considerable interest comes from the E. coli bacterium. It is known as the NarI sequence and contains the bases G1-G2-C-G3, where C is the base cytosine. Surprisingly, G3 is a mutational hotspot when bound by certain aromatic amine carcinogens while G1 and G2 are not. The underlying reason for this difference has been a mystery and is of great importance because it is a paradigm for mutational hotspots, such as in the p53 gene, which are found mutated in many human tumors.
We have elucidated the structure of a DNA duplex containing the NarI sequence linked at G1, G2, or G3 with a model aromatic amine carcinogen known as 2-aminofluorene (AF), using a combination of high-resolution NMR solution studies and molecular mechanics computations. These studies have revealed a striking difference in structure when the carcinogen damage is at G3, compared to G1 or G2. When the AF is at G1 or G2, it resides preponderantly in the major groove of an unperturbed B-DNA double helix. However, when the AF is at G3, it resides half the time in a position where it is inserted into the helix, causing the damaged guanine to be displaced from its normal helix-inserted position. It is plausible that this structural distortion, if also present during DNA replication in the cell, could be responsible for the failure of the DNA to replicate normally when the hotspot is damaged, leading to the mutatagenic consequence.
Significance
This work is the first delineation of structural distinctions between mutagenic hotspots and coldspots, revealing how subtle differences in base sequence can produce remarkable differences in structure that can explain the hotspot phenomenon.
Publications
Mao B., Gu Z., Hingerty B. E., Broyde S. and Patel D. J. N. d. Solution structure of the aminofluorene [AF]-intercalated conformer of the syn [AF]-C8-dG adduct opposite dC in a DNA duplex. Biochemistry, In Press.
Mao B., Gu Z., Hingerty B. E., Broyde S. and Patel D. J. N. d. Solution structure of the aminofluorene [AF]-external conformer of the anti [AF]-C8-dG adduct opposite dC in a DNA duplex. Biochemistry, In Press. |